

In the wake of a joint investigation by KHN and CBS News into a dental appliance that multiple lawsuits allege caused grievous harm to patients, the FDA has begun looking into the product, the Anterior Growth Guidance Appliance, or AGGA, according to a former agency official.

This story also ran on CBS News. It can be republished for free.

A dental device called AGGA has been used on about 10,000 patients without FDA approval or proof that it works. In lawsuits, patients report irreparable harm. The AGGA’s inventor and manufacturer have denied all liability in court.
Additionally, KHN and CBS News have learned that the Las Vegas Institute, a training company that previously taught dentists to use the AGGA, now trains dentists to use another device it has described as “almost exactly the same appliance.” That one is called the Anterior Remodeling Appliance, or ARA.
The FDA’s interest in the AGGA was revealed by Cara Tenenbaum, a former senior policy adviser in the agency’s device center who has said the FDA should investigate the product, which has been fitted on more than 10,000 dental patients, according to court records.
Tenenbaum said that after KHN and CBS News published their report, she was contacted by “very concerned” FDA officials who said they have begun “looking into” the AGGA but have yet to determine how much legal authority the agency has to regulate it.
“The FDA is looking at what authorities they may have around this device — what they may be able to do,” Tenenbaum said. “Now, of course, whether or not this device is FDA regulated, it still needs to be safe.”

KHN and CBS News have reviewed online messages verifying that an FDA official has communicated with Tenenbaum about the AGGA. The FDA declined to comment on the AGGA or confirm whether it was evaluating the device.
The KHN-CBS News investigation of the AGGA involved interviews with 11 patients who said they were hurt by the device — plus attorneys who said they represent or have represented at least 23 others — and dental specialists who said they’d examined patients who had experienced severe complications using the AGGA. The investigation also found no record of the AGGA being registered with the FDA, despite the agency’s role in regulating medical and dental devices.
The AGGA’s inventor, Tennessee dentist Dr. Steve Galella, has said in a sworn court deposition that the device was never submitted to the FDA, which he believes doesn’t have jurisdiction over it. Tenenbaum has said the lack of registration is “incredibly problematic” because that is one method by which the FDA collects reports of a device’s negative effects.
She encouraged anyone who has witnessed complications from the AGGA to assist the FDA by submitting a report through its MedWatch portal.
“Whether that’s a dentist, an orthodontist, a surgeon, a patient, family member, or caregiver,” Tenenbaum said, “anyone can and should submit these reports so the FDA has a better understanding of what’s happening.”

Kragulj is one of at least 20 AGGA patients who have in the past three years filed lawsuits against Galella and other defendants claiming the AGGA did not — and cannot — work. The plaintiffs allege that instead of expanding their jawbones, the AGGA left them with damaged gums, loose teeth, and eroded bone.
Some allege in lawsuits they will lose teeth because of the device and added in interviews that they no longer have enough healthy bone to replace those teeth with dental implants.
“I can take my finger now and I can literally wiggle my front teeth,” said Melanie Pappalardi, 28, who said she wore an AGGA for a year and filed a lawsuit in Indiana. “I can’t bite into absolutely anything.”
The plaintiffs do not allege in their lawsuits that Galella treated them but that he or his company consulted with each of their dentists about their AGGA treatment.
Galella has declined to be interviewed by KHN and CBS News. His attorney, Alan Fumuso, has said in a written statement that the AGGA “is safe and can achieve beneficial results.”
All the AGGA lawsuits are ongoing. Attorneys for Galella and his company, the Facial Beauty Institute, have denied liability in court filings. Johns Dental Laboratories settled one lawsuit for an undisclosed amount but continues to fight allegations in the other cases. The Las Vegas Institute, which previously held AGGA classes for dentists and promoted the device on Facebook, denied liability in court and has a pending motion to end claims in one lawsuit in which it is named as a defendant.
In a sworn deposition filed in that lawsuit, Las Vegas Institute CEO Dr. Bill Dickerson said that in 2020 he began to question the claims about what the AGGA could accomplish, then severed all ties with Galella.
However, that same year the Las Vegas Institute pivoted to the Anterior Remodeling Appliance, or ARA, according to Facebook posts by Dickerson. Dickerson has said in multiple Facebook posts over the past three years that the AGGA and ARA are very similar, including in a June 2021 post that described ARA as “almost exactly the same appliance.” He also said that most dentists associated with the Las Vegas Institute have switched to the ARA, which is made by a different dental laboratory than AGGA’s manufacturer.
“Different lab. Same thing,” Dickerson said in another Facebook post.
The Las Vegas Institute did not respond to requests for comment on the ARA. Institute attorney William Schuller has previously declined to discuss the ARA.
Dental specialists who have warned about the AGGA said they are also alarmed by the ARA.
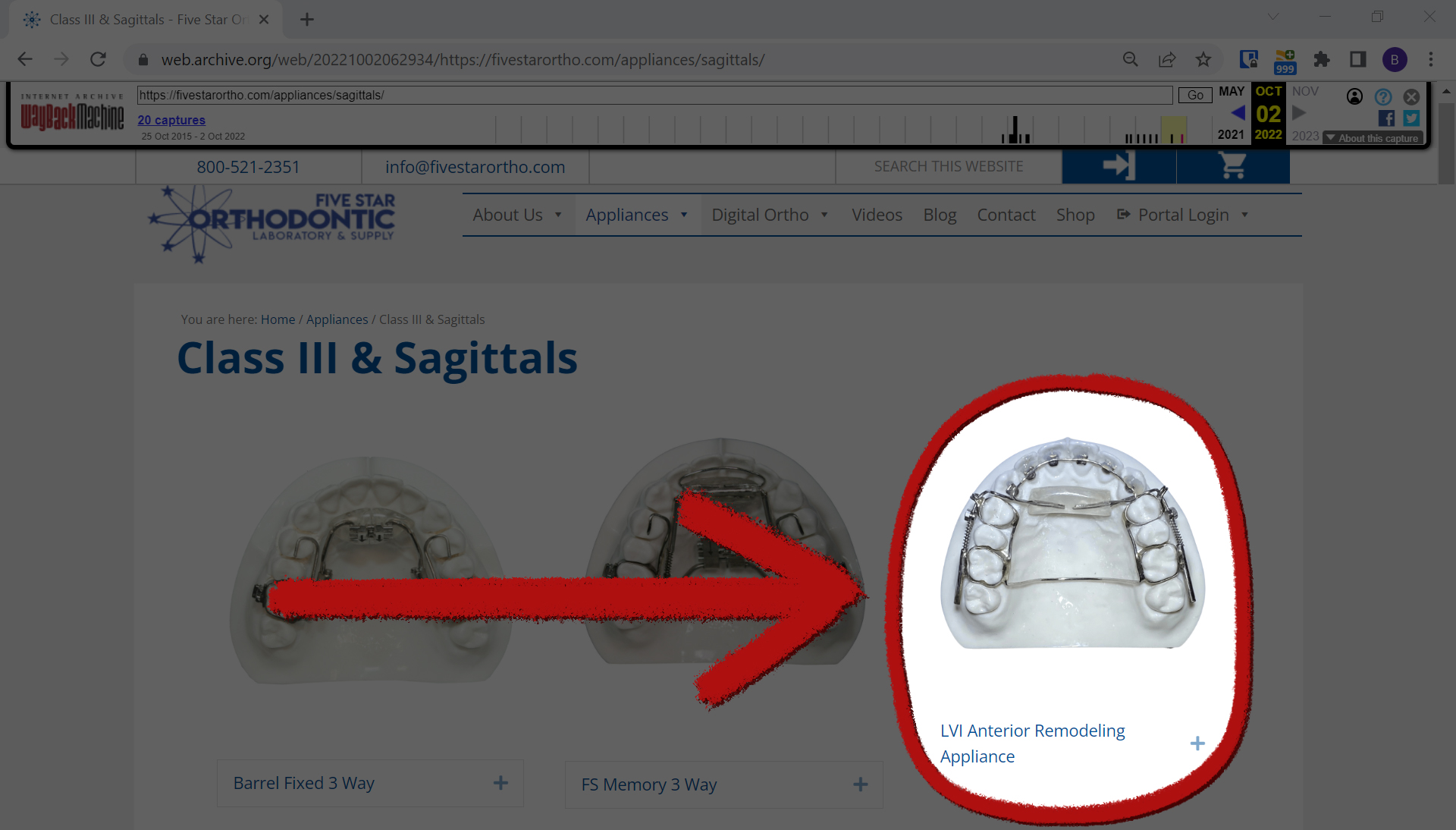
Dr. Kasey Li, a California maxillofacial surgeon, and Dr. George Mandelaris, a Chicago-area periodontist, each of whom have said they’ve examined multiple patients harmed by the AGGA, said after looking at a photo of the ARA from the manufacturer’s website, it appears to be very similar to the AGGA.
“It is very similar to the AGGA,” Mandelaris said in an email. “Almost identical.”